間葉系幹細胞(MSC)のカウント Counting of mesenchymal stem cells
 |
 |
 |
 |
NucleoCounter®は、吸引した脂肪組織や骨髄穿刺液 などから得られた間葉系幹細胞(MSC)の生存率と総細胞数を効果的に測定するための堅牢で正確な方法を提供します。
細胞治療のためのMSCのカウント
再生治療における間葉系幹細胞の重要性
MSCは様々な疾患の治療に使用されており、特に骨疾患、心臓疾患、筋骨格系疾患、循環器に影響を与える疾患で注目されています。これらの細胞は、患者(自家細胞)、あるいは遺伝的に異なる他人(他家細胞)から作製されます。MSC研究に大きな関心が集まっているのは、これらが骨、脂肪、軟骨、筋肉、神経細胞に分化できることが発見された(1-3)からであり、再生治療における興味深い対象となっています。MSC移植の臨床応用での可能性は、組織再生(4,5)から移植片対宿主病などの免疫疾患(6)の治療まで多岐にわたります。さらに、MSCは抗アポトーシス性や血管新生性のサイトカイン、成長因子などを分泌し、心筋梗塞後の治癒過程(7)や創傷治癒(8)をサポートする能力があります。MSCの貴重な供給源は、吸引された脂肪組織や骨髄組織、臍帯血、胎盤、 Wharton´s Jellyなどです(図1)。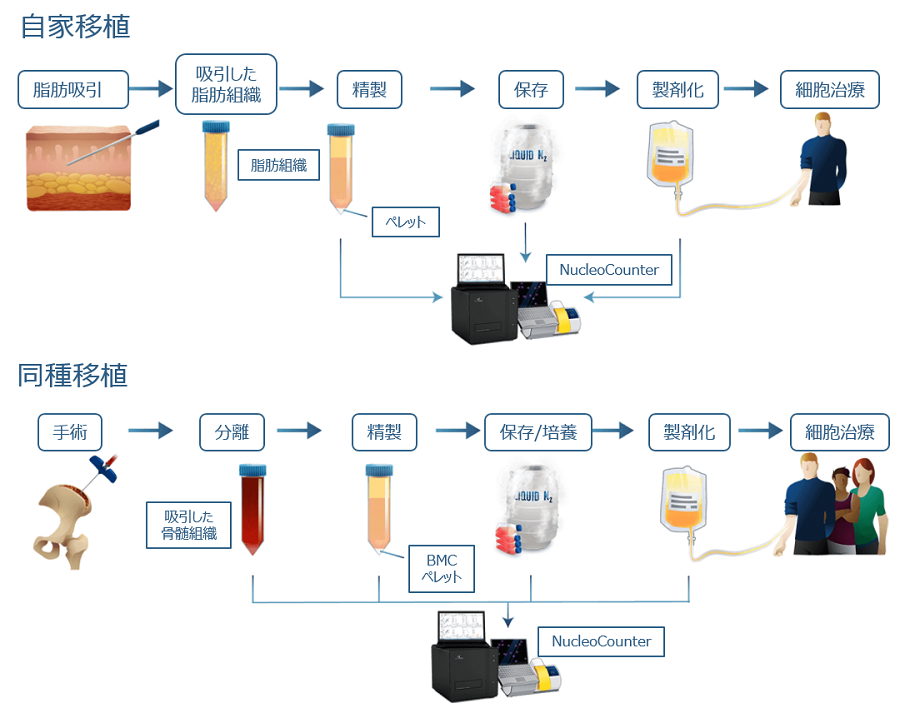
MSCは、脂肪由来の場合は脂肪吸引後の間質血管画分(SVF)からの分離、または骨髄穿刺で採取した骨髄組織の精製により得られます。採取したMSCは、直接あるいは、培養を行い自家/他家細胞療法用として長期保存されてから使用されます。これらすべての様々な生産ステップでNucleoCounter®を使用することにより、細胞数と生存率を確実に測定できます。
脂肪組織由来の間葉系幹細胞の正確なカウント
脂肪由来のMSCは増殖性が高く、脂肪生検や脂肪吸引後の間質血管画分(SVF)から簡単に分離できます(9-11)。この画分には、さまざまな種類の細胞、例えば MSC、間質細胞、内皮細胞、脂肪細胞、赤血球、脂肪滴やミセルが含まれています(12)。細胞治療用途では、SVFは直接患者の治療に使用されます。総細胞数と生存率を正確に定量することは、細胞播種などの下流のアプリケーションに極めて重要です。多くのセルカウント手法では、細胞とアーティファクトの区別に失敗したり、オペレーター間で大きなばらつきが生じたりします。この問題を克服するために、NucleoCounter®はヒト、動物の脂肪由来MSCの総細胞数と生存率を決定するための効果的なプロトコールを提供します(13-18)。
NucleoCounter®を使ったSVFの総細胞の決定では、細胞懸濁液を使用機種に応じた試薬で処理することで、細胞やその他の膜封入粒子を溶解し、例えばVia1-Cassette™を使って細胞核をDAPI染色します(図2)。DAPIがDNAに特異的に結合することにより、NucleoCounter®は核DNAを含む細胞のみをカウントするため、赤血球や血小板などのDNAを含まない細胞だけでなく、細胞片(デブリ)、ミセル、細胞外小胞、脂肪滴などのSVFでよく観察されるアーティファクトをカウントすることを回避できます(18)。
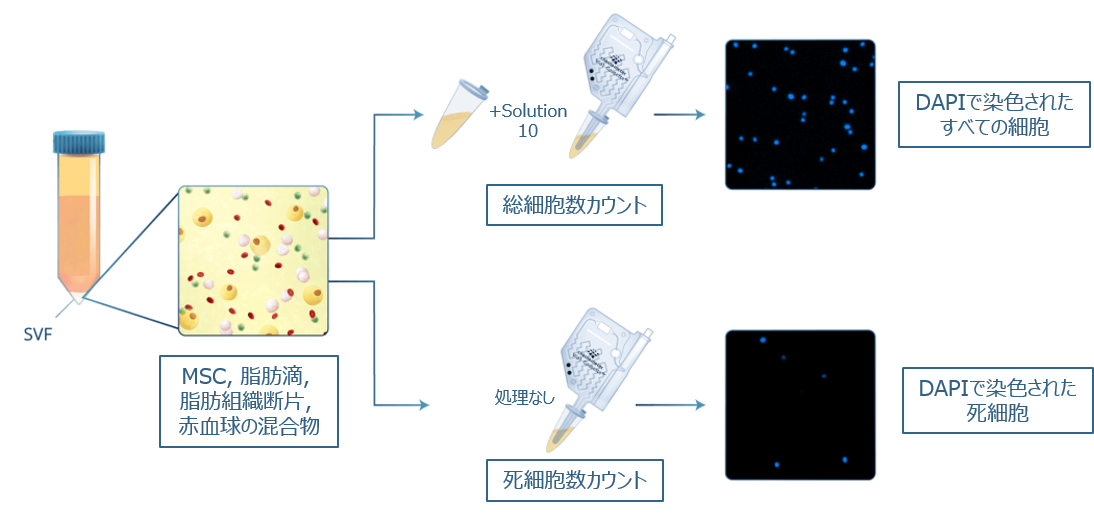
様々な細胞種に加えて、SVFにはミセル、脂肪滴、赤血球、細胞外小胞などのアーティファクトが含まれています。SVF分画中の総有核細胞数には、Solution 10(または、Lysis 1やReagent A100 and B)を添加することで細胞膜を溶解し、細胞核を溶液中に懸濁させます。DAPIで蛍光染色された細胞核の総数がNucleoCounter®で検出され、総細胞数としてカウントされます。また、前処理を行わないサンプルをDAPI染色することにより、死細胞数がカウントされます。
アプリケーションノート(PDF)
 Via-1カセットとSolution 10を使用した凝集細胞の生存率と総細胞数カウント
Via-1カセットとSolution 10を使用した凝集細胞の生存率と総細胞数カウントReagent A100 and BとVia-1カセットを使用した凝集細胞のカウント
骨髄由来間葉系幹細胞の正確なカウント
骨髄由来のMSCを分離するために、骨髄組織または骨髄液を採取します(19、20)。骨髄サンプル中のMSC細胞数と生存率を、従来のセルカウンターやマニュアル計数で直接決定することは、サンプル中に含まれる高濃度な赤血球により非常に困難です。骨髄穿刺液中のMSCを直接的、簡単かつ信頼性高くカウントするために、NucleoCounter®はサンプル中の赤血球を処理するための迅速なプロトコールを提供します(図3.)。希釈した骨髄穿刺液に溶血バッファーを添加することにより、赤血球が溶解して、内部の高濃度ヘモグロビンが溶液中にリリースされて希釈されます。その後、サンプルはアクリジンオレンジ(AO)とDAPIによって蛍光染色されます。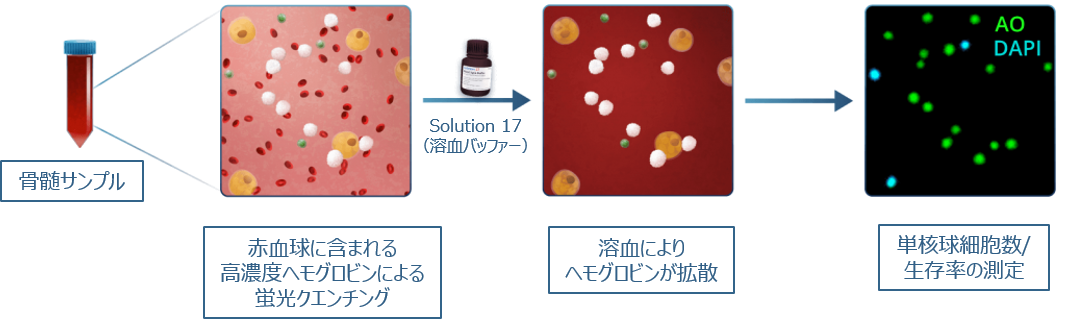
骨髄吸引液にSolution 17を加え、短時間のインキュベーションを可能にすることにより、赤血球が溶解され、有核細胞のみがアクリジンオレンジ(すべての細胞)とDAPI(死細胞)で染色されます。 その後、NucleoCounter®を使用して有核細胞を確実に検出します。
アプリケーションノート(PDF)
 全血をサンプルとした有核細胞の生存率と総細胞数カウント
全血をサンプルとした有核細胞の生存率と総細胞数カウント
細胞治療分野におけるユーザーの声
NC-200™ を当社の自動セルカウンターとして選択したことで、FDA の申請や臨床現場でのスタッフのトレーニングに要する時間を短縮することができました。
 “We need to use a cell counting device that is FDA-compliant and capable of counting Adipose-derived Regenerative Cells rapidly and efficiently. After testing several options, we found that the NucleoCounter® NC-200™ device fits our required criteria and consistently delivers rapid, reproducible and reliable results.
We did not have to perform any additional validation studies related to cell counting and viability due to the FDA’s familiarity with the instrument. Selecting the NC-200™ as our automatic cell counter saved us time during the FDA application as well as during training of the staff at the clinical sites. The quality of the device and the excellent customer service that ChemoMetec provides has been a key factor in the successful initiation of our clinical program.”
“We need to use a cell counting device that is FDA-compliant and capable of counting Adipose-derived Regenerative Cells rapidly and efficiently. After testing several options, we found that the NucleoCounter® NC-200™ device fits our required criteria and consistently delivers rapid, reproducible and reliable results.
We did not have to perform any additional validation studies related to cell counting and viability due to the FDA’s familiarity with the instrument. Selecting the NC-200™ as our automatic cell counter saved us time during the FDA application as well as during training of the staff at the clinical sites. The quality of the device and the excellent customer service that ChemoMetec provides has been a key factor in the successful initiation of our clinical program.”Glenn Winnier, PhD, Chief Scientic Officer
InGeneron社:脂肪組織から脂肪由来再生細胞(ADRC)を分離するためのプラットフォームを開発している細胞治療機器メーカーです。
市場にあるどのトリパンブルー/ヘマサイトメーター自動セルカウンターよりもNC-200をお勧めします。
 “The NC-200 provides us with accurate and repeatable results we can trust. The automated counter is capable of accurately counting the SVF cells ー even though many of the cells are stuck in clumps. This is extremely important for our procedures and the NC-200 can provide us with trustworthy repeatable results. It is easy to use, fast, and requires no maintenance/calibration. I would recommend the NC-200ー over any trypan blue/hemacytometer automated cell counters on the market.”
“The NC-200 provides us with accurate and repeatable results we can trust. The automated counter is capable of accurately counting the SVF cells ー even though many of the cells are stuck in clumps. This is extremely important for our procedures and the NC-200 can provide us with trustworthy repeatable results. It is easy to use, fast, and requires no maintenance/calibration. I would recommend the NC-200ー over any trypan blue/hemacytometer automated cell counters on the market.”Jason Pacchiarotti, Compliance and Quality Manager
VentCell社:親会社であるPrimeGen Biotech社のヒト細胞研究に基づいた動物用治療法を開発するための「One Health」アプローチを採用し、獣医学分野で使用するためのFDA承認の細胞ベースの治療法を開発することに焦点を当てた再生医療企業です。
引用文献
1. Dominici, M., et al., Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 2006. 8(4): p. 315-317.
2. Makino, S., et al., Cardiomyocytes can be generated from marrow stromal cells in vitro. Journal of Clinical Investigation, 1999. 103(5): p. 697-705.
3. Arthur, A., et al., Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 2008. 26(7): p. 1787-1795.
4. Horwitz, E.M., et al., Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(13): p. 8932-8937.
5. Kawada, H., et al., Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood, 2004. 104(12): p. 3581-3587.
6. Zhao, K., et al., Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation, 2015. 21(1): p. 97-104.
続きはこちらをクリック
7. Hahn, J.-Y., et al., Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes and therapeutic efficacy for myocardial infarction. Journal of the American College of Cardiology, 2008. 51(9): p. 933-943.
8. Schnabel, L.V., et al., Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. Journal of Orthopaedic Research, 2009. 27(10): p. 1392-1398.
9. Zuk, P.A., et al., Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell, 2002. 13(12): p. 4279-4295.
10. Mizuno, H., M. Tobita, and A.C. Uysal, Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells, 2012. 30(5): p. 804-810.
11. Gimble, J.M., A.J. Katz, and B.A. Bunnell, Adipose-derived stem cells for regenerative medicine. Circulation Research, 2007. 100(9): p. 1249-1260.
12. Astori, G., et al., “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. Journal of Translational Medicine, 2007. 5: p. 55-55.
13. Kølle, S.-F.T., et al., Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. The Lancet, 2013. 382(9898): p. 1113-1120.
14. Araña, M., et al., Preparation and characterization of collagen-based ADSC-carrier sheets for cardiovascular application. Acta Biomaterialia, 2013. 9(4): p. 6075-6083.
15. Kazantseva, J., et al., Alternative splicing targeting the hTAF4-TAFH domain of TAF4 represses proliferation and accelerates chondrogenic differentiation of human mesenchymal stem cells. PLoS ONE, 2013. 8(10): p. e74799.
16. Choi, J.S., et al., In vitro expansion of human adipose-derived stem cells in a spinner culture system using human extracellular matrix powders. Cell and Tissue Research, 2011. 345(3): p. 415-423.
17. Suga, H., et al., IFATS collection: Fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells, 2009. 27(1): p. 238-249.
18. Miyazaki, T., et al., Isolation of two human fibroblastic cell populations with multiple but distinct potential of mesenchymal differentiation by ceiling culture of mature fat cells from subcutaneous adipose tissue. Differentiation, 2005. 73(2): p. 69-78.
19. Meirelles, L.d.S., P.C. Chagastelles, and N.B. Nardi, Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science, 2006. 119(11): p. 2204-2213.
20. Kuznetsov, S.A., et al., Circulating skeletal stem cells. The Journal of Cell Biology, 2001. 153(5): p. 1133-1140.
21. Heathman, T.R.J., et al., Expansion, harvest and cryopreservation of human mesenchymal stem cells in a serum-free microcarrier process. Biotechnology and Bioengineering, 2015. 112(8): p. 1696-1707.
22. Heathman, T.R.J., et al., Serum-free process development: improving the yield and consistency of human mesenchymal stromal cell production. Cytotherapy, 2015. 17(11): p. 1524-1535.
消耗品
| 品番 | 容量 | ||
|---|---|---|---|
| 941-0012 | Via1-Cassette™ | 100個/箱 | 生存率・細胞数測定 (NC-200・NC-3000) |
| 941-0024 | Via2-Cassette™ | 100個/箱 | 生存率・細胞数測定 (NC-202) |
| 910-3010 | Solution 10 Lysis buffer | 100mL | 2-Step Cell Cycle, 全細胞数測定(凝集細胞) |
| 910-3017 | Solution 17 | 25mL | Blood Lysis Buffer |
| 910-0010 | Lysis 1, 100 ml | 100mL | 細胞処理試薬 (Total Count) |
アプリケーションノート


